A semi-solid cell is a battery technology in which the electrolyte portion appears in a semi-solid state, intermediate between a solid-state battery and a traditional liquid battery. This design provides higher energy density and safety because the solid state portion reduces the risk of leakage and thermal runaway while maintaining a high ionic conductivity, resulting in a better balance of battery performance.
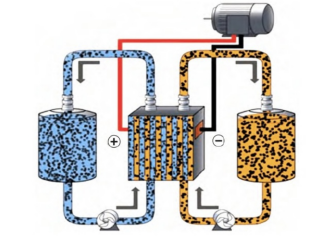
Semi-solid state battery is a new electrochemical energy storage technology, its electrode suspension mainly consists of active materials, conductive additives and electrolyte. The positive and negative electrode suspensions of the battery are installed in two liquid storage tanks, and the liquid delivery pump is utilized to circulate the suspension between the battery reactor and the external liquid storage tank. Inside the battery reactor, the positive and negative suspensions are separated by an ionic diaphragm. When charging, the ions inside the battery move from the positive to the negative terminal, and the electrons move from the positive to the negative terminal through the external circuit; while when discharging, the ions and electrons move in the opposite direction to the charging.
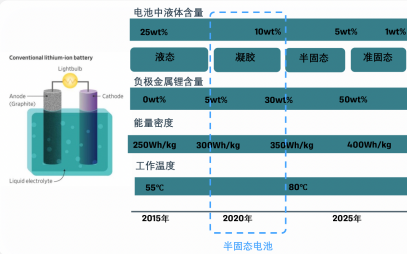
Advantages of semi-solid-state battery cells
The advantage of semi-solid batteries is that the energy storage capacity and power can be regulated independently, i.e., the energy storage capacity is determined by the size of the liquid storage tank, and the power is determined by the size of the battery reaction chamber. In addition, the utilization of positive and negative electrode materials is improved compared to conventional batteries, and the electrolyte in the electrode suspension is easy to replace or replenish. By optimizing the composition of the electrode suspension and the cell structure, the electrochemical performance of the battery can be further improved and its overall cost can be reduced.
This design combines the characteristics of solid-state and liquid batteries and has the following significant features:
(1) Safety: Semi-solid batteries are safer compared to traditional liquid batteries. Since the electrolyte part is solid, it is not easy to leak, reducing the risk of battery explosion. Rule out the danger of traditional lithium batteries in the following situations: ① work under high current lithium dendrites may occur, which may puncture the diaphragm leading to short circuit damage. ② The electrolyte is an organic liquid, and the tendency of side reactions, oxidative decomposition, gas generation, and combustion will be intensified at high temperatures.
(2) High energy density: Semi-solid batteries can achieve higher energy density, which means that they can store more energy in the same volume and weight, and provide longer usage time.
(3) Fast Charging: Semi-solid state cells overcome the problem of slow charging of traditional solid state batteries with better charging performance, allowing faster charging of the battery.
(4) Environmentally Friendly: Compared to traditional batteries, the materials used in semi-solid batteries are more environmentally friendly, reducing the negative impact on the environment.
Due to the above advantages of semi-solid batteries, it has a broad application prospect in the fields of electric vehicles, portable electronic devices and renewable energy storage.
What are the advantages of semi-solid batteries over traditional liquid batteries?
Higher safety: Compared to traditional liquid batteries, semi-solid batteries utilize a gel-like or porous semi-solid electrolyte, which reduces the risk of battery leakage and combustion, and therefore provides a higher level of safety.
Higher energy density: Compared to solid-state batteries, the electrolyte in semi-solid batteries typically has better ionic conductivity, allowing the battery to store more energy in the same volume, increasing energy density.
Better fast charging performance: Semi-solid batteries have better ionic conductivity, which can support faster charging speeds and shorten charging times.
Good low-temperature performance: Semi-solid batteries are more stable at low temperatures than traditional liquid batteries, with less degradation of battery performance.
Eco-friendly: Semi-solid batteries usually use inorganic materials or renewable resources as electrolyte, which reduces the impact on the environment and is more environmentally friendly.
Better cycle life: Since semi-solid batteries avoid the flow of liquid electrolyte, there is less corrosion and buildup inside the battery, resulting in a better cycle life.
Overall, semi-solid batteries have certain advantages over traditional liquid batteries and solid state batteries in terms of safety, energy density, charging performance, low-temperature performance, environmental friendliness, and cycle life.
How does a semi-solid battery work?
Migration of ions in solid-liquid electrolyte mixtures during charging and discharging, leading to energy storage and release.
The main components of a semi-solid battery include two electrodes (usually positive and negative) and a semi-solid electrolyte. The positive and negative electrodes are separated by the semi-solid electrolyte, forming an electrochemical reaction area.
During charging and discharging, the positive electrode releases electrons while the negative electrode absorbs them. Meanwhile, in the semi-solid electrolyte, ions will move between the positive and negative electrodes. Specifically:
Charging process:
During charging, an external power source supplies current to the battery, which causes metal ions in the positive electrode material (e.g., lithium ions) to begin to de-embed and release electrons. These electrons flow back to the negative electrode through the external circuit, completing the charging process of the battery. At the same time, the metal ions in the positive electrode material are transported to the negative electrode in the semi-solid electrolyte and embedded in the negative electrode, completing the ion transport in the battery.
Discharge process:
During discharge, an external circuit connects the load and electrons flow from the negative electrode to the positive electrode to provide energy to the load. At the same time, metal ions in the negative electrode material begin to de-embed and are transported to the positive electrode in the semi-solid electrolyte. At the positive electrode, these metal ions are embedded in the positive material, completing the discharge process in the battery.
Throughout the charging and discharging process, the semi-solid electrolyte plays the role of ionic conduction, which allows effective ion transfer between the positive and negative electrodes, thus completing the charging and discharging reactions of the battery.
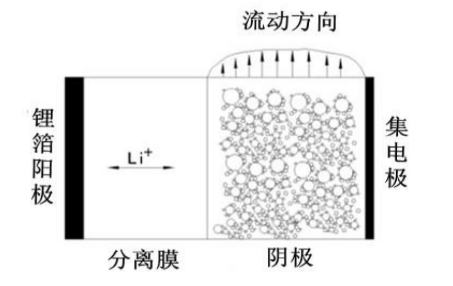
Semi-solid liquid flow battery working principle diagram
In which areas do semi-solid state batteries have promising applications?
Electric Vehicles: Semi-solid state batteries have the potential to be an important energy storage solution in electric vehicles due to their high energy density and fast charging performance. This battery technology can provide longer range and shorter charging time, thus promoting the popularization and development of electric vehicles.
Wearables: As smartwatches, smart bands, and other wearable devices become more popular, there is a growing demand for small, lightweight, and high energy density batteries. The high energy density and safety of semi-solid state batteries make them an ideal energy source for wearable devices.
Mobile devices: Mobile devices such as smartphones and tablets require reliable, high-performance batteries. The fast-charging performance and long cycle life of semi-solid state batteries make them an attractive power option for mobile devices.
Energy Storage Systems: The high energy density and reliability of semi-solid state batteries make them a potential choice for home and industrial energy storage systems. These systems can be used to balance energy supply and demand, storing renewable energy sources such as solar and wind.
Aerospace: In the aerospace sector, the light weight and high performance of batteries are critical to the performance of aircraft and satellites. Semi-solid state batteries have the advantage of providing higher energy density and better safety performance, and therefore have the potential to be widely used in the aerospace sector.
Wearable Medical Devices: The medical industry has a high demand for small, safe and reliable batteries to support the use of various wearable medical devices. The characteristics of semi-solid state batteries give them potential for application in this area.
In fact, there are many more scenarios where it can be used due to its following characteristics :Improved safety, high temperature resistance, high energy density, fast charging capability
Long cycle life, design flexibility
The fundamental defects of lithium iron phosphate
(1) In the sintering process during the preparation of lithium iron phosphate, there is a possibility that iron oxide will be reduced to monomeric iron under a high-temperature reducing atmosphere. Monatomic iron can cause micro-short circuit of the battery, which is a taboo substance in the battery.
(2) Lithium iron phosphate has some performance defects, such as very low vibration density and compaction density, resulting in low energy density of lithium-ion batteries. The low-temperature performance is poor, and even its nanosizing and carbon coating have not solved this problem.
(3) The preparation cost of the material and the manufacturing cost of the battery are high, and the battery yield is low and the consistency is poor. Although the nanosizing and carbon coating of lithium iron phosphate improves the electrochemical performance of the material, it also brings other problems, such as the reduction of energy density, the increase in the cost of synthesis, poor processing performance of the electrode and harsh environmental requirements. Although the chemical elements Li, Fe and P in lithium iron phosphate is very rich, the cost is also lower, but the preparation of lithium iron phosphate product cost is not low, even if the previous R & D costs are removed, the material process costs coupled with the higher cost of preparing batteries, will make the unit of energy storage power cost is high.
(4) Poor product consistency. From the point of view of material preparation, the synthesis reaction of lithium iron phosphate is a complex multiphase reaction, with solid-phase phosphates, iron oxides, and lithium salts, plus precursors of carbon and reducing gas phase. In this complex reaction process, it is difficult to ensure the consistency of the reaction.


